Table of Contents
Introduction
One of the legitimate concerns that many parents have about adhering to the routine childhood vaccine schedule recommended by the US Centers for Disease Control and Prevention (CDC) is that many vaccines contain aluminum, which is used as an “adjuvant”.
On a page of its website titled “Adjuvants and Vaccines”, the CDC explains the purpose of the aluminum by saying, “An adjuvant is an ingredient used in some vaccines that helps create a stronger immune response in people receiving the vaccine. In other words, adjuvants help vaccines work better.”
To reassure the public, the CDC states that “Aluminum salts, such as aluminum hydroxide, aluminum phosphate, and aluminum potassium sulfate have been used safely in vaccines for more than 70 years.” The CDC says that all aluminum-containing vaccines “are tested for safety and effectiveness in clinical trials before they are licensed for use in the United States” and are “continually monitored by CDC and FDA once they are approved.”
The page has been recently updated to acknowledge that a CDC study published in September 2022 found “a positive association between cumulative vaccine‑associated aluminum before age 24 months and persistent asthma at age 24 through 59 months among children with and without eczema.” For children with eczema, the finding was a 26 percent increased risk of asthma for every 1,000 micrograms of aluminum received by vaccination.
The CDC, however, downplays these findings by describing the study as having found a “possible” association, even though the increased risk of asthma in children with and without eczema was statistically significant and the study authors themselves acknowledge that a “positive association” was found. The CDC further describes the association between aluminum-containing vaccines and an increased risk of asthma as merely a “potential” safety signal rather than acknowledging it to be an existential safety signal.
Thusly downplaying the increased risk of asthma associated with aluminum exposure from vaccines, the CDC persists in its policy course and its longstanding claim that “the amount of aluminum exposure in people who follow the recommended vaccine schedule is low and is not readily absorbed by the body.”
To support its claim that the cumulative levels of aluminum that children are exposed to by its routine childhood vaccine schedule is “low” and that the aluminum is readily eliminated from the body without causing toxic effects, the CDC cites a study by researchers from the Food and Drug Administration (FDA). That study, conducted by Robert J. Mitkus et al. and published in the journal Vaccine in 2011, is titled “Updated aluminum pharmacokinetics following infant exposures through diet and vaccination”.
The FDA likewise cites the study by Mitkus et al. as having shown that “the risk to infants posed by the total aluminum exposure received from the entire recommended series of childhood vaccines over the first year of life is extremely low.”
However, that study does not support the government’s claims about vaccine safety. Indeed, the claims made by the CDC and FDA serve to illustrate the complete untrustworthiness of the “public health” establishment.
In truth, aluminum is a known neurotoxin that accumulates in tissues and organs, including the brain, and that plausibly causes neurological harms to children at the cumulative levels that children are exposed to by the CDC’s routine childhood vaccine schedule.
Concerns about the potential toxic harms to children have been raised in the medical literature, but the CDC cherry-picks data to support its policies, as illustrated by how it cites the FDA study while willfully ignoring other research demonstrating the serious errors made by Mitkus et al. in their analysis.
Indeed, for these “public health” agencies to rely on that FDA study as proof that the aluminum in vaccines is “safe” amounts to outright scientific fraud.
The Purpose of Aluminum Adjuvant
As noted, the CDC states that the purpose of an adjuvant is to help vaccines to “work better” by creating “a stronger immune response”.
What the CDC means is that inclusion of an adjuvant causes the vaccine to elicit a more inflammatory immune response, which results in the induction of a higher level of circulating antibodies. This in turn enables pharmaceutical companies to obtain the level of antibodies required by the FDA to be considered sufficiently immunogenic and therefore to obtain licensure for their products.
For example, in its 1997 “Summary Basis of Approval” document for GlaxoSmithKline’s diphtheria, tetanus, and acellular pertussis (DTaP) vaccine, which is sold under the trade name Infanrix and includes 0.5 milligrams (mg)—or 500 micrograms (μg)—of aluminum hydroxide as an adjuvant, the FDA noted that:
In the absence of a laboratory or serologic correlate of protection to pertussis, the sponsor was asked to demonstrate that the antibody responses to the pertussis antigens in US children receiving Infanrix as a three dose primary series were comparable to the responses in Italian and German children immunized in the efficacy studies.
The FDA stressed again that none of the assays used for the measurement of antibodies “has been shown to correlate with protection” against pertussis, or whooping cough. The current package insert for Infanrix similarly notes that “a serologic correlate of protection for pertussis has not been established”, meaning that there is no specific level of antibodies that has been shown to correlate with immune protection against the disease.
Depending on the pathogen and the individual, having a high level of antibodies does not necessarily mean that the person is protected against the disease, and having a low level of antibodies does not necessarily mean that the person does not have immunity. The FDA nevertheless accepted measurements of antibody levels as a surrogate measure of immunity for the purposes of vaccine licensure.
Lack of Placebo-Control Groups and ‘Non-Specific Effects’ of Vaccines
To reassure the public, the CDC states that aluminum adjuvants have been used “safely” in vaccines for more than 70 years. But that statement is practically meaningless. Just because aluminum has been used an adjuvant in vaccines for so many decades does not mean that these vaccines have not caused adverse effects in children. This statement of the CDC’s simply begs the question.
The CDC also says that it continually monitors vaccine safety after vaccines obtain FDA licensure, but postmarketing surveillance data is no substitute for properly designed safety studies.
As noted by the authors of an article published in the Journal of Pharmacy and Pharmacology in March 2015, “The most widely used adjuvant, alum, has been used for nearly 90 years, yet its mechanism of action remains poorly understood. In addition, while alum produces a powerful antibody Th2 response, it does not provoke the cellular immune response required for the elimination of intracellular infections or cancers.”
The authors of the CDC study finding an association between adjuvanted vaccines and asthma acknowledged the possibility that “exposure to aluminum through vaccination could produce an immune profile biased toward Th2 and away from T helper 1 cell (Th1) immune responses.” This skewing of the immune system toward an antibody response at the cost of imbalanced cellular immunity could possibly “increase the risk of allergic diseases such as asthma”.
Indeed, as noted by the authors of a review published in 2005 in the European Respiratory Journal, “Substantial experimental evidence now supports the notion that allergic diseases are characterized by a skewing of the immune system towards a T-helper cell type-2 (Th2) phenotype.” As similarly observed by the authors of a review titled “Unbalanced neonatal CD4+ T-cell immunity” and published in Frontiers in Immunology in 2014, the development of asthma is known to be associated with “a Th2-skewed immune response”.
The CDC nevertheless reassures the public that all aluminum-containing vaccines “are tested for safety and effectiveness in clinical trials before they are licensed for use in the United States”.
However, that doesn’t mean that these vaccines were tested for safety and efficacy in randomized placebo-controlled trials.
Looking again at Infanrix as an example, the FDA’s Summary Basis for Approval document reveals that no inert placebo was used. Instead, the FDA determined that the DTaP vaccine was “safe” based on a comparison of adverse events after administration of the older whole-cell pertussis vaccine (DTP), which was withdrawn from the market in the US precisely because it was more “reactogenic”, resulting in parental concerns about the vaccine causing injury to children.
It was largely vaccine injury lawsuits against DTP manufacturers that led to the US government in 1986 passing a law granting broad legal immunity to the manufacturers against vaccine injury lawsuits and establishing the Vaccine Injury Compensation Program (VICP) to shift the financial burden for vaccine injuries away from the pharmaceutical industry and onto the taxpaying consumers.
The DTP vaccine is still widely used in developing countries, where studies into the “non-specific effects” of vaccines, which term refers to beneficial or detrimental unintended consequences of vaccination, have found the vaccine to be associated with an increased rate of childhood mortality. As leading researchers in the field put it in a 2017 study published in the Lancet’s EBioMedicine journal:
It should be of concern that the effect of routine vaccinations on all-cause mortality was not tested in randomized trials. All currently available evidence suggests that DTP vaccine may kill more children from other causes than it saves from diphtheria, tetanus or pertussis. Though a vaccine protects children against the target disease it may simultaneously increase susceptibility to unrelated infections.
This detrimental non-specific effect has been found to be true for “non-live” vaccines generally, which are the types of vaccines that typically contain aluminum adjuvant, and the effect is particularly pronounced for female children. (Attenuated “live” virus vaccines like the measles vaccine do not require an adjuvant to induce a sufficient immune response since the antigen exposure from the vaccine more closely resembles exposure to the whole viable virus from natural infection.)
The FDA document for Infanrix curiously describes one efficacy trial for the DTaP vaccine as having been “placebo-controlled”, but there is no mention of a placebo control group. Instead, the “placebo” that was evidently used was the DTP vaccine.
The problem of biologically reactive substances being commonly used in lieu of a true placebo is reflected in the title of a study published in Annals of Internal Medicine: “What’s in Placebos: Who Knows? Analysis of Randomized Controlled Trials”. That review pointed out that most studies “did not disclose the composition of the study placebo” and that “no regulations guide placebo composition, which can influence study results.”
Naturally, using another aluminum-containing vaccine as a “placebo” by which to determine the alleged safety of another aluminum-containing vaccine will conceal the true background rate of adverse events following vaccination, enabling the pharmaceutical product to be rather meaninglessly labeled “safe” by the FDA.
In lieu of randomized placebo-controlled trials demonstrating the long-term safety of aluminum-containing vaccines, researchers must rely on toxicological studies or postmarketing observational studies.
However, as acknowledged in 2015 by the director of the CDC’s Immunization Safety Office, Frank DeStefano, “no large epidemiological studies have specifically examined associations between health outcomes” and aluminum adjuvants.
Hence the CDC’s reliance on the FDA study by Mitkus et al. to support its claim about the alleged safety of aluminum-adjuvanted vaccines.
The FDA’s ‘Safe Limit’ for Aluminum Exposure vs. the Vaccine Schedule
The Environmental Protection Agency (EPA) has established a maximum contaminant level for aluminum in drinking water of 0.05–0.2 milligrams per liter (mg/L), and the FDA’s own maximum “safe” limit for drinking water is 0.2 milligrams per liter, or 200 micrograms per liter (µg/L).
Under federal regulations, for large volume drugs or therapies administered parenterally, meaning not via the gastrointestinal tract (such as intramuscular or intravenous injection), the FDA has established a maximum “safe” limit of 25 micrograms per liter (µg/L).
These products must include a warning in their package inserts that the aluminum content “may be toxic” and that “Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 µg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.”
The Agency for Toxic Substances and Disease Registry (ATSDR), a department that is administratively overseen by the director of the CDC and operates alongside the CDC and FDA under the auspices of the Department of Health and Human Services (HHS), has also defined a “minimal risk level” (MRL) for aluminum, which is “an estimate of daily human exposure to a substance that is likely to be without an appreciable risk of adverse effects (noncarcinogenic) over a specified duration of exposure.”
The “safe” level for “chronic-duration oral exposure (365 days or longer) to aluminum”, according to the ATSDR, is 1 mg/kg/day. It was this estimated “minimal risk level” that served as the basis for the calculations done by Mitkus et al.
The first point to make about the CDC’s deceptiveness is how it characterizes that study as having examined “the amount of aluminum exposure in people who follow the recommended vaccine schedule”, when in fact the study was limited to only the vaccine doses administered to infants during their first year of life. Children following the CDC’s schedule continue to receive aluminum-containing vaccines after their first birthday, so the CDC’s statement is misleading. That is the least of the CDC’s deceptiveness, however.
Using the schedule as of 2011, Mitkus et al. looked at “potential combinations of FDA-licensed routine childhood vaccines” to “determine the maximum doses of aluminum that a child might receive over the course of a year.”
On the first day of their lives, infants typically receive 0.25 mg of aluminum from the Hepatitis B (HepB) vaccine.
At two months of age and again at four months of age, they might receive a dose of HepB, DTaP, inactivated poliovirus vaccine (IPV), Haemophilus influenzae type B (Hib) vaccine, and pneumococcal vaccine (PCV), which depending on the brands chosen could result in up to 1.2 mg of aluminum exposure at each of those two doctor visits.
At six months of age, children might receive HepB, DTaP, IPV, and PCV, resulting in up to 0.975 mg of aluminum exposure.
At their one-year checkup, children might receive Hib, PCV, and Hepatitis A (HepA) vaccine, resulting in 0.6 mg of aluminum exposure.
The study provides the following table summarizing the maximum amount of aluminum that infants were being exposed to during their first year of life by the CDC’s vaccine schedule as of 2011.
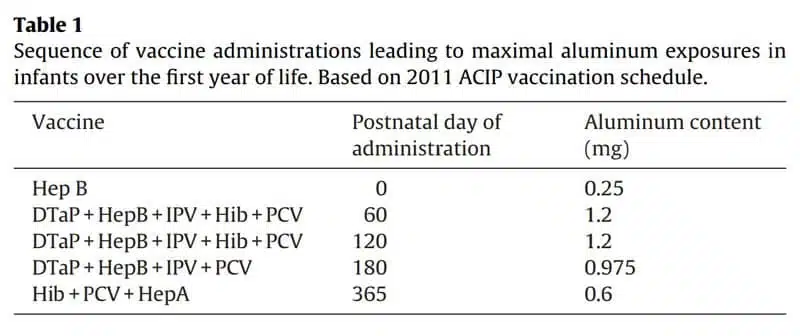
The FDA and CDC assure us that this cumulative exposure to aluminum children following the schedule might receive during their first year of life—up to 4,225 micrograms—represents a “low” amount of aluminum that is completely “safe”.
However, as noted in a review of available evidence on aluminum adjuvants and autism published in the Journal of Trace Elements in Medicine and Biology in April 2021, that study “was seriously flawed”. We will now examine the study’s major errors in detail to illustrate how the government’s claim of safety is scientifically fraudulent.
The FDA’s Falsified ‘Minimal Risk Level’ for Aluminum
As noted, the FDA researchers’ conclusion that the amount of aluminum children are exposed to from vaccines administered during the first year of life pose an “extremely low risk to infants” was premised on a “minimal risk level” set by the ATSDR of 1 mg/kg/day, and exposures below this level are considered to be “safe” by the FDA.
Using that “safe” limit, the FDA researchers calculated the “body burden” of aluminum from either aluminum phosphate (AlPO4) or aluminum hydroxide (Al[OH]3) adjuvants. The resulting two graphs they present suggest that the cumulative levels of aluminum that children are exposed to by the vaccine schedule is well below the FDA’s “safe” limit.
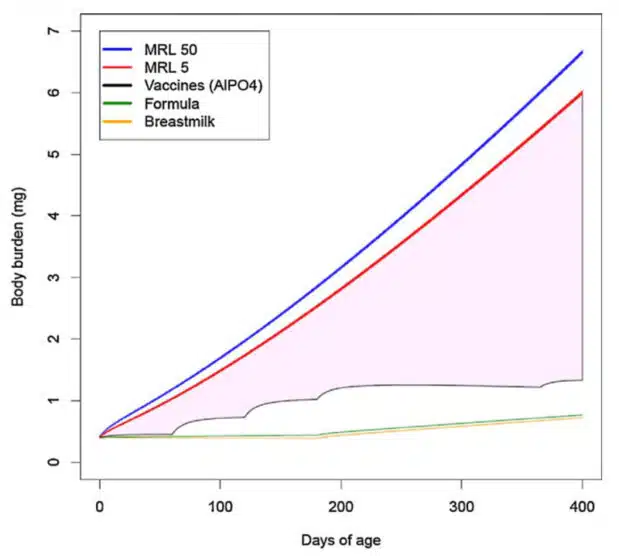
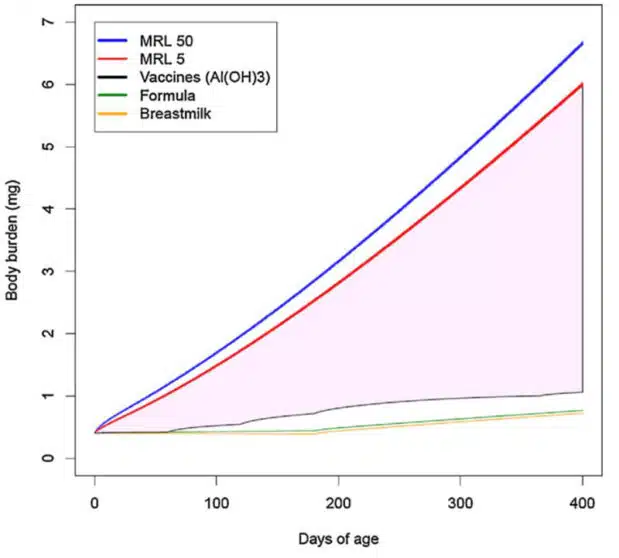
However, the CDC fails to inform the public that the minimal risk level used by FDA researchers was calculated based on a single study of ingested aluminum lactate, a soluble form of aluminum, in adult mice.
Major problems with the FDA study were observed in an anonymously authored critique published on the website Vaccine Papers in July 2016 and titled “Debunking Aluminum Adjuvant, Part 2: FDA’s Flawed Study of Al Adjuvant Toxicity (Mitkus 2011)”. As noted in that article, the minimal risk level used by the FDA researchers “is based on outdated and wrong science.” It was “derived from a ‘No Observed Adverse Effect Level’ (NOAEL) measured in an animal feeding study”. The article explains:
However, there is a fatal problem: 26 mg/kg/day is not a NOAEL (safe dosage) for animals. The NOAEL of 26 mg/kg/day (ingested) is the foundation for the Mitkus analysis, and it is wrong. Scientific studies report adverse effects (e.g., brain injury, cognitive impairment, and brain inflammation) from ingested (and water-soluble) aluminum dosages of 3.4, 4, 5.6, 6, 10, and 20 mg/kg/day.
Those studies are reviewed in a separate article on Vaccine Papers titled “The Foundation for Al Adjuvant Safety Is False”.
In a paper published in the Journal of American Physicians and Surgeons in 2016, Neil Z. Miller, author of the book Miller’s Review of Critical Vaccine Studies, similarly explains several “major flaws in the FDA’s analysis”, starting with the use of a minimal risk level that had already been falsified by other animal studies (emphasis added):
To determine an aluminum intake “minimal risk level” (MRL) for humans, a single animal study was used. This study found that mice could receive up to 26 milligrams of aluminum per kilogram of body weight per day (26 mg/kg/day) with no adverse effects. After considering differences between mice and humans (and other factors), this number was reduced to create a margin of safety, and an MRL of 1 mg/kg/day was established for humans, including infants. But there is a problem: 26 mg/kg/day is not a safe amount of aluminum for animals. Several studies confirm that animals are harmed by much lower quantities of aluminum—3.4 to 6.1 mg/kg/day—and at least three of these studies were published before the FDA paper in 2011, so the FDA study was fallacious at its inception.
In a review titled “Critical analysis of reference studies on the toxicokinetics of aluminum-based adjuvants”, published in the Journal of Inorganic Biochemistry in December 2017, Jean-Daniel Masson et al. similarly explained that “Mitkus’ study suffers from a number of important biases.” The first problem is that “An inappropriate oral MRL was used to define the safety curve.” The “oral MRL” used by the FDA researchers “sets the safety curve too high.” Citing studies finding adverse effects in animals at much lower levels of exposure, including a 2017 study observing adverse effects with an exposure of 1.5 mg/kg/day, the review authors remark:
By using the “official” oral MRL, Mitkus therefore set the safety curve at a much higher level. This level was overestimated by a factor of up to 17.3 (i.e., 26/1.5) when the most recent study was taken into account. It should be noted that the 1.5 mg/kg/day is not even a NOAEL since effects have been documented at this dose.
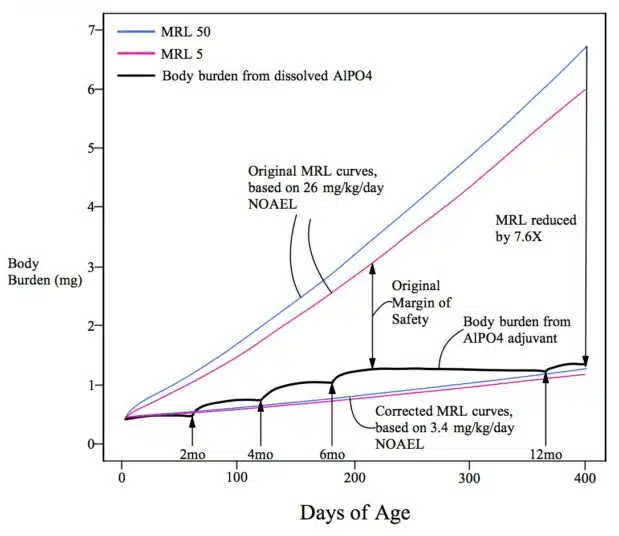
The review authors present two corrected graphs originally published by the author of the Vaccine Papers article showing how, “even if one uses higher experimental NOAEL levels for calculation, e.g., 3.4 mg/kg/d, the safety limit is reached (hydroxide) or overstepped (phosphate) by Al from vaccine adjuvants.”
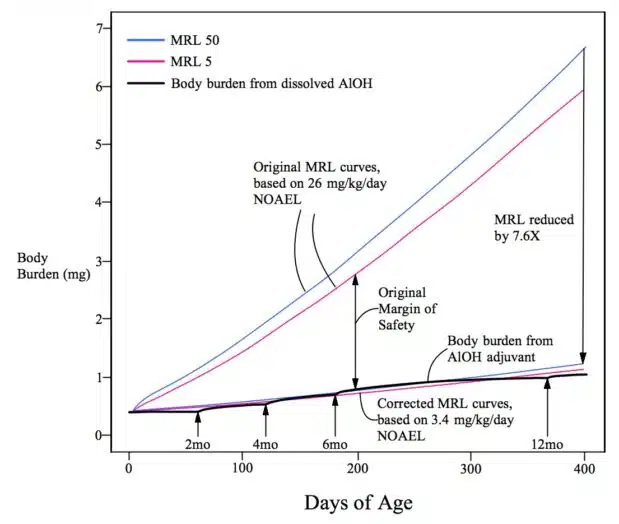
Injected vs. Ingested Aluminum
It gets worse. As noted, the minimal risk level used by the FDA researchers as the basis for their calculations was derived from a study of ingested and not injected aluminum.
As the FDA knows perfectly well, “the gastrointestinal tract acts as an efficient barrier to the absorption of aluminum, and relatively little ingested aluminum actually reaches body tissues”, whereas “parenterally administered drug products containing aluminum bypass the protective mechanism of the gastrointestinal tract” and is “deposited in human tissues.”
Mitkus et al. similarly acknowledged (emphasis added):
Potential aluminum exposures associated with vaccine administration, however, are different from dietary exposures to aluminum, since aluminum in vaccines does not have to pass through the walls of the gastrointestinal tract, which is a significant barrier to systematic aluminum absorption. Rather, it is expected that the whole amount of aluminum in the adjuvant will be absorbed from muscle into the blood following vaccination, albeit at some rate over time.
. . . Dietary exposure to aluminum (usually as the citrate) results in small amounts of aluminum being absorbed from the gut (<1%) and reaching the bloodstream.
Yet, they curiously offered no comment on how the “safe” level of exposure they adopted as the basis for their analysis was based on ingested aluminum in rodents, which could reasonably be interpreted as indicative of intent to perpetrate scientific fraud.
As Miller comments:
The MRL for humans is derived from dietary aluminum fed to mice. But infants are injected with aluminum. Injected aluminum bypasses the gastrointestinal tract and has unique toxic properties compared to aluminum that is ingested. To determine the safety of injected aluminum, scientists must conduct experiments with injected—not ingested—aluminum.
Ignoring the Toxicity of Aluminum Particles
What perhaps explains the authors’ failure to acknowledge the inapplicability of the adult rodent study used to determine the adopted “minimal risk level” for human infants is the fact that they only considered the amount of aluminum that dissolves and is absorbed into the blood, from where it can then be processed through the kidneys and excreted in urine, as contributing to the “body burden” of aluminum toxicity; they completely ignored the amount of particulate aluminum that remains in tissues and organs.
This is a “critical error”, as noted by the author of the Vaccine Papers article:
The Mitkus analysis is a reasonable way to estimate the toxicity and retention of ingested water-soluble aluminum (Al3+ ions). But it cannot establish safety of low-solubility, persistent aluminum adjuvant particles. . . .
The MRL is derived from a feeding experiment with water-soluble aluminum lactate, not insoluble and persistent aluminum adjuvant particles. The safety of injected aluminum adjuvant can only be proven by experiments with injected aluminum adjuvant (which comprise insoluble and persistent particles). Studies of ingested, water-soluble aluminum are not a good substitute. Scientific studies have established that injected aluminum adjuvant has unique toxic properties and ways of moving around the body (“kinetics”) that are not the same as water-soluble aluminum.
Compounding the error of using a study on ingestion of a soluble form of aluminum is the additional “fatal flaw” of ignoring the toxicity of aluminum particles remaining in the body:
Mitkus assumes that aluminum adjuvant has zero toxicity while in particulate form. The “body burden” of Mitkus does not include the Al adjuvant particles. In Mitkus’s analysis, only Al3+ ions (released by dissolving adjuvant particles) are toxic.
This is why the Al adjuvant curve has an upward slope after each vaccination date. . . .
Al adjuvant nanoparticles are not harmless or inert. They are biologically active, and cause inflammation and immune system activation. That is why Al adjuvant is used in most vaccines. Adjuvant particles travel to tissues that can be injured by inflammation, like the brain.
The article presents this graphic illustration of the fatal flaws of the FDA’s study along with the following comment:
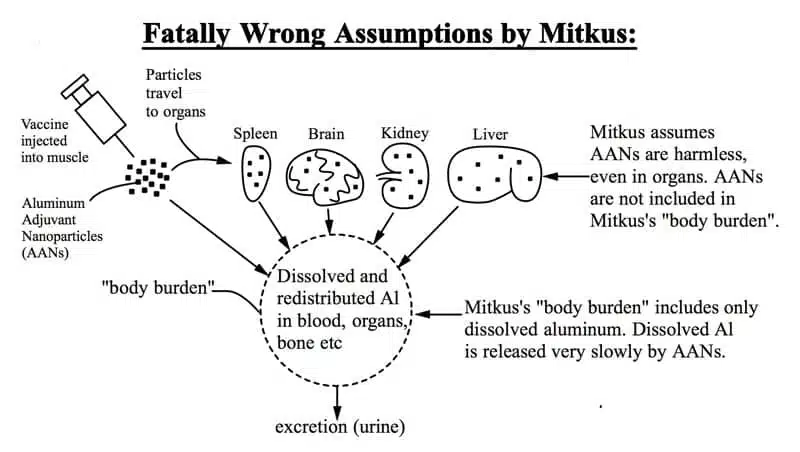
Above: Injected Al adjuvant nanoparticles travel to distant organs where they remain for years, causing inflammation. Mitkus assumes the particles have zero toxicity, even when present in sensitive organs like the brain. Mitkus erroneously assumes only the dissolved Al3+ is toxic. There is no evidence for this assumption, and conclusive evidence from animal experiments and studies of other nanoparticle toxicity research that it is wrong.
Neil Z. Miller similarly observes that “aluminum nanoparticles can be transported by monocyte-lineage cells to draining lymph nodes, blood and spleen‑and may also penetrate the brain.” Nevertheless, the FDA “assumes, without evidence, that these poorly biodegradable aluminum nanoparticles, which have been detected in body organs up to a year after vaccination, are harmless, and they are not calculated by the FDA as part of the aluminum ‘body burden’ until they dissolve.”
Masson et al. also criticize Mitkus et al. for failing to consider the toxicity of particulate aluminum, remarking (emphasis added):
In its particulate form, Al is rapidly captured and then transported at a distance by immune cells. The comparison of the chemical toxicity of Al ions, such as those absorbed at the intestinal level, and the particulate toxicity of Al salts injected [intramuscularly] is therefore nonsense. This is evidenced by the atypical dose-response curve of the neurotoxic effects of Al hydroxide, with cerebral transfer of aluminum and a clinical effect selectively observed for low dose, which approximates those described in particulate toxicology. Strictly speaking, MRL used for vaccine risk modeling should be defined on the basis of animal experiments carried out with Al adjuvants, monitored for their particle parameters to be in accordance with those of the vaccines, and injected [intramuscularly], rather than studies with soluble forms of Al (chloride or lactate) added to food or drinking water.
When mentioning “the atypical dose-response curve of the neurotoxic effects of Al hydroxide”, they were referring to a study by Guillemette Crépeaux et al. titled “Non-linear dose-response of aluminium hydroxide adjuvant particles: Selective low dose neurotoxicity”, which was published in the journal Toxicology in 2017.
As the authors of that Toxicology study noted, mouse experiments had shown “capture and slow transportation” of aluminum particles “by monocyte-lineage cells from the injected muscle to lymphoid organs and eventually the brain.” Their findings indicated that aluminum adjuvant “injected at low dose in mouse muscle may selectively induce long-term [aluminum] cerebral accumulation and neurotoxic effects.”
The authors understatedly noted that “comparing vaccine adjuvant exposure to other non-relevant aluminium exposures, e.g., soluble aluminium and other routes of exposure, may not represent valid approaches.”
As an example, they cited Mitkus et al., whose model of infant retention was based on “aluminium retention rate observed after intravenous injections of traceable soluble aluminium citrate”. The FDA’s model was “based on the hypothesis that aluminium adjuvants are solubilized by citrate ions in muscle interstitial fluid, without any consideration of quick adjuvant cellular uptake and systemic long term diffusion of adjuvant agglomerates.” (Emphasis added.) They concluded by suggesting, again understatedly, that “reevaluation” of the safety of aluminum adjuvants is required.
Coming back to the critique of the FDA study by Masson et al., they noted that the mathematical model developed by Mitkus et al. also incorporated an estimate of aluminum retention in children based on a study of a single adult given an intravenous injection of aluminum citrate, a soluble form of aluminum.
Mitkus et al. pointed out that “it is expected that aluminum is not cleared from the blood of infants as quickly as that of adults”, and they therefore attempted to adjust their model for the higher retention of infants.
However, as Mitkus et al. also acknowledge, “For vaccines, the injection is intramuscular, the aluminum is in an insoluble form (e.g., as the phosphate or hydroxide of aluminum), and muscle at the site of injection is considered to be a storage depot for aluminum.” They acknowledge that one of the limitations of their study is that their “retention function” for aluminum is “based on results for only one person”, whereas ideally it should be based on pharmacokinetic data in multiple infants.
To estimate their rate of absorption of aluminum, which represents the aluminum particles that “are solubilized by citrate ions in the interstitial fluids of muscle” and absorbed into the blood, they used data from a study investigating the absorption of aluminum administered intramuscularly into two rabbits for each of the two types of aluminum adjuvant used in childhood vaccines (aluminum hydroxide and aluminum phosphate).
They acknowledged this limitation of their study, but the relevant takeaway they emphasized is that there is a “slow release of aluminum adjuvant from the site of injection into the systemic circulation”. Since they assumed that only the amount of aluminum absorbed into the blood contributed to the body burden of aluminum toxicity, they interpreted this as reassuring.
For example, they note that if 100 percent of the injected aluminum was instantaneously absorbed into the blood (which would also be the case for intravenous as opposed to intramuscular injection), then there would be “brief ‘excursions’ of bodily aluminum levels above the MRL following vaccination”. They reasoned that since the absorption of aluminum in the blood occurs slowly rather than instantaneously, the risk of toxicity is lower.
However, that reasoning is again fallacious since the assumption that the amount of particulate aluminum remaining in the body is non-toxic is false.
Masson et al. also comment on this aspect of the FDA study:
Mitkus et al. took into account the slow absorption (solubilization) of Al from adjuvants shown by Flarend, and, in so-doing, found a seemingly high safety margin. To build their model, Mitkus et al. reasoned as follows: since the blood absorption of aluminum was 51% for phosphate adjuvant at 28 days after the injections in the Flarend study, it would take 28 more days to absorb the whole injected dose of adjuvant (total 56 days). Similarly since blood absorption of Al was 17% for Al hydroxide at 28 days, complete absorption would take 137 additional days (total 165 days). The calculated cumulative amount of aluminum absorbed from vaccines was significantly higher than the dietary Al uptake . . . but remained below the safety level for Al phosphate, and very largely below for Al hydroxide. The author’s conclusion is that the Al from vaccines is unlikely to have a significant influence on Al body burden of the infant’s organism, implying a good safety of Al adjuvants from 0 to 12 months.
However, the FDA study “suffers from a number of important biases.” As already noted, the FDA researchers used an inappropriate minimal risk level that had already been falsified by other studies showing adverse effects in animals at much lower levels of exposure. They also ignored the toxicity of aluminum particles remaining in the body rather than being absorbed into the blood. Additionally, they incorporated a misinterpretation of the rabbit study by Flarend et al. into their model (emphasis added):
Like Flarend before him, Mitkus et al. seemingly considered the only the soluble Al has toxic potential. His estimation of the duration of complete translocation of Al from the injected site to blood . . . is based on a simplistic calculation (see above) not taking into account that Flarend’s curves suggest that the termination of Al translocation to plasma is either underway (phosphate) or nearly achieved (hydroxide) on the 28th day (see above). The corollary of this over simplistic calculation is an underestimation of the bio-persistence time of Al in particular form. Histological studies carried out after [intramuscular] injection of Al hydroxide showed that particulate Al and the granulomas it induces, are still detectable in the injected muscle after months in animal studies and several years (up to 12 years) in adult patients with chronic post-vaccine fatigue syndrome. Although genetic factors might explain the low intracellular solubilization of Al hydroxide in susceptible individuals, the Mitkus underestimation of the stability towards dissolution of aluminum adjuvants is certain and significant.
The aluminum from vaccines also does not remain at the injection site, but “can migrate away from the muscle in its particulate form.”
The FDA study reasoned that it is “reassuring” that “the long-term storage depot” for aluminum solubilized from the injection site was likely to be primarily in the bones and not organs like the brain. With respect to this reasoning, Masson et al. further comment (emphasis added):
Considering soluble Al only, Mitkus thought that “long-term storage depot (of Al solubilized from the injected site), is likely to be skeletal and not a more sensitive soft organ system is reassuring”. This reassuring assumption did not take into account the fate of particulate Al. In the same way, a recent study performed in premature infants vaccinated at the age of 2 months, only focused on soluble Al detectable in body fluids: the authors curiously felt “reassuring” the fact that they did not notice elevation of Al in serum and urine 24 h after administration of vaccines containing a total dose of 1200 μg Al (about 200 μg/kg). The absence of both detectable absorption and rapid elimination of Al from adjuvants rather represents a legitimate reason of concern, since, as a corollary, it likely indicates systemic persistence of immunostimulating and neurotoxic Al particles translocated to lymphoid organs and potentially reaching the brain.
A study by Grant McFarland et al. published in the Journal of Trace Elements in Medicine and Biology in December 2019 similarly criticizes the Mitkus et al. study by describing it as having been “thoroughly excoriated as mere mathematical journey based on the faulty assumption that oral doses of aluminum (of a different form from those found in vaccines) in adult mice could be used to predict the impact of [adjuvanted vaccines], as if those data can be transformed via math into relevant data for injected forms in human adults and children.”
As McFarland et al. summarize (emphasis added):
In short, the Mitkus paper based an assessment of the safety of water-insoluble forms of injected aluminum in human adults on a single study of dietary water-soluble forms of aluminum in adult mice. This study, cherry-picked from among many by ATSDR, was used to claim that the doses of aluminum used on humans are safe. . . . Studies that use oral citrate solution administration of aluminum . . . are of no relevance for study of the toxicity of aluminum types from vaccines . . . . Animal studies are further limited due to their inability to address the hypothesis of genetic variation [in] aluminum tolerance in humans.
With relevance for how the FDA researchers cited the rabbit study as offering reassurance of the safety of aluminum adjuvants in vaccines, Masson et al. similarly comment (emphasis added):
Rather than talking about the reassuring nature of these results, an inverse conclusion should have been made by the authors from a vaccine safety perspective, highlighting the low dissolution elimination of Al adjuvants, especially the hydroxide-based adjuvant, and the need for further long-term studies on a larger number of animals. The regulatory agencies themselves would have been well advised to order complementary toxico-kinetic studies in order to avoid the propagation of hazardous information on the rapid elimination of Al adjuvants, especially after they had become aware of subsequent studies showing phagocytosis, intracellular persistence, distance migration and neurotoxicity of Al adjuvants.
Conclusion
Now consider again how the CDC cites the FDA study by Mitkus et al. as having “shown” that aluminum from vaccines “is not readily absorbed by the body.” The inescapable logical conclusion is that this claim from the “public health” establishment is dangerous disinformation.
The CDC says that the purpose of the aluminum in vaccines is to help the vaccines “work better”, but what that really means is that it enables the manufacturers to achieve the level of circulating antibodies required to obtain FDA licensure, regardless of whether or not that level of antibodies correlates with immune protection against disease.
The CDC says that aluminum has been used in vaccines for more than 70 years, but it certainly does not follow that therefore the cumulative levels of aluminum that children are exposed to today by the CDC’s recommended schedule are “safe”.
The CDC says that each adjuvanted vaccine undergoes clinical trials prior to licensure, but the FDA allows vaccine manufacturers to forego randomized placebo-controlled trials in favor of comparing rates of adverse events between individuals receiving the experimental vaccine and another aluminum-containing product.
The CDC says that government agencies monitor postmarketing surveillance data to ensure vaccine safety, but such data is no substitute for randomized placebo-controlled trials, and these government agencies simply have no credibility given their inherent conflict of interest and historical record of mischaracterizing the science to suit their policy aims.
Dr. Frank DeStefano, senior author of the recent CDC study admitting an association between adjuvanted vaccines and asthma, had in 2015 acknowledged the absence of observational studies specifically examining associations between health outcomes and aluminum-containing vaccines.
The CDC says that “the amount of aluminum exposure in people who follow the recommended vaccine schedule is low and is not readily absorbed by the body”, but that claim is based on a fatally flawed FDA modeling study that based its conclusion on an already falsified “minimal risk level” for aluminum exposure and on the FDA’s willful ignorance of the toxicity of particulate aluminum from vaccines that remains in the tissues and organs rather than being dissolved and absorbed into the blood, where it can then be processed through the kidneys and excreted in urine.
As a simple thought experiment, consider what the response would be from the “public health” establishment and mainstream media if the findings of such a study instead supported the conclusion that the amount of aluminum children are exposed to from vaccines causes harm. Somehow, every study suggesting vaccines can cause harms is fatally flawed, while every study whose findings can be interpreted—or misinterpreted—as supportive of government vaccine policy is accepted unquestioningly as yet more proof of vaccines being “safe”, regardless of any methodological limitations or huge disparities between authors’ conclusions and their actual findings.
In sum, the CDC’s claim that the aluminum in vaccines has been proven “safe”, including for infants, is an outright lie and indicative of how the government is willing to perpetrate scientific fraud to sustain its existing policy recommendations. The fact that the CDC so grossly mischaracterizes the state of scientific knowledge about aluminum toxicity is a clear illustration of its untrustworthiness. Clearly, the government is more concerned with serving the financial interests of the pharmaceutical industry than with public health.



A great reason to be an anti-vaxxer. If I had kids, knowing what I know now about vaccines, I would take them and run for the hills. If aluminum was beneficial to humans, I am sure the body would offer some kind of biological mechanism to make it helpful. The same could be said about all the other junk they add to vaccines…many ingredients we never are told are present. WHY?
Informed consent does not occur.
Vaccines today prescription drugs tomorrow
That seems to be the business model.
Don’t forget the mercury ☿ too
Quite right. Synergistic effects of mercury with aluminum, too.
Killer journalism as always. I had to look up whether the other studies mentioned in the Vaccine Papers articles also gave the AlCl orally or injected it into the rodents. They administered it orally. Though that is certainly implied in your article, I would have found it even clearer to state it explicitly.
Thanks for checking and confirming that the other studies also involved oral administration.
Somehow, in the process of you quoting it, a typo was introduced into the Vaccine Papers quote:
<>
It became “does not included that”. Just FYI, feel free to delete this comment once resolved.
Thank you for pointing out the typo for me! I’m not sure how that happened either. Must have just gotten distracted in the middle of typing it out. I have corrected it.
I found myself in a convo re: aluminum in zeolite, and remembered your article – this one – from Twitter years ago, and knew I needed to find it and share. I remembered you (you’re awesome btw), and many long threads of you educating the ppl against the lies and liars. You won every point they came with lol
But I couldn’t remember your name… somehow found you by searching. Very cool. Thanks for being here, Jeremy.
Hi Gina,
Thanks for letting me know you found my article helpful. I really appreciate the kind words of encouragement! It’s also good to know you were able to find me by searching since I know that Google prejudices my site in its search result rankings, as I demonstrate in this article:
https://www.jeremyrhammond.com/2023/01/17/censorship-and-the-legacy-medias-misinformation-monopoly/