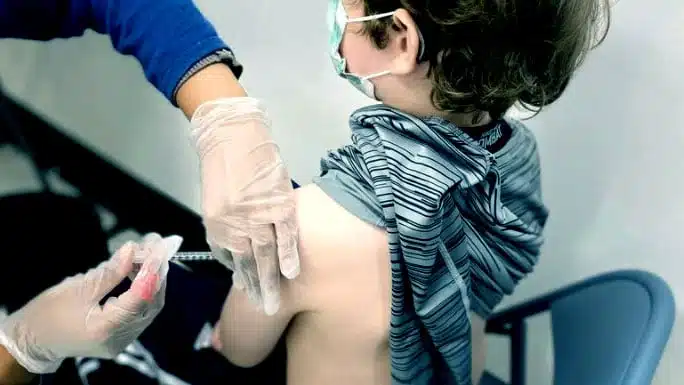
On the morning of December 7, 2021, I saw a new advertisement from the Michigan Department of Health and Human Services (MDHHS) on the social media platform Nextdoor promoting the Pfizer-BioNTech COVID-19 vaccine for children as young as age five. The ad directs parents to get more information at a webpage of the Michigan state government.
In this video (on Rumble and Odysee), I review and fact check the ad and the information provided to parents by state health officials, showing how supposedly trustworthy “public health” officials, rather than empowering parents with the knowledge they need to make an informed choice, are misleading and blatantly lying to parents to persuade them to comply with the policy goal of achieving high vaccine uptake.
Deceptions I cover include the following:
- The rationale for vaccinating young children presented in the MDHHS ad falsely assumes that the risk for them is the same as the risk to middle aged adults.
- How the MDHHS claims that the Pfizer-BioNTech COVID-19 vaccine and the Comirnaty vaccine are the same thing when in fact they are two distinct products, and Comirnaty is not even currently available in the US.
- How the MDHHS similarly communicates to parents that there is equivalence between FDA “authorization” and FDA “approval”.
- How the MDHHS uses its obfuscation over those product and regulatory distinctions to mislead parents into believing that the Pfizer vaccine has been FDA approved for children aged 5 – 11.
- How the MDHHS falsely claims that the vaccine authorized for emergency use in children aged 5 – 11 has undergone the same type of study and regulatory review as FDA approved vaccines.
- How the MDHHS fails to disclose to parents that if their child is harmed by the vaccine, they cannot sue the manufacturer because the government has granted legal immunity to the pharmaceutical companies for COVID-19 vaccines being made available under emergency use authorization.
- How the MDHHS misleads parents into believing that tens of thousands of children aged 5 – 11 were included in phase three clinical trials for the Pfizer vaccine when in truth the number was 2,268 (1,517 in the experimental group and 751 in the placebo control group). (Only at the very end of the document does MDHHS acknowledge that only a few thousand children in this age group were included, not tens of thousands as they initially imply.)
- How the clinical trial data for children aged 5 – 11 measured only risk of symptomatic SARS-CoV-2 infection, not severe disease, hospitalization, or death.
- How the data show an absolute risk reduction of just 2% against symptomatic infection, indicating that in order to prevent a single case of symptomatic infection, 51 children need to be vaccinated.
- How for children with evidence of prior infection, which is evidence of natural immunity, the risk reduction was 0% (no children previously infected had symptomatic infection).
- How seroprevalence data presented by the CDC to the FDA’s advisory committee that recommended authorization of the Pfizer vaccine for children aged 5 – 11 indicated that 42% of children had already been infected through June 2021, which is likely an underestimate both because more children have since been infected and because the antibody test used likely missed some cases of prior infection.
- How the MDHHS implies that vaccinating children aged 5 – 11 will contribute to the development of herd immunity when in truth the effectiveness of the vaccines against infection wanes rapidly and fully vaccinated individuals can transmit the virus to others.
- How the MDHHS tells parents that it is safe for them to get their children aged 5 – 11 to get the COVID-19 vaccine coadministered with other vaccines including flu shots, even though the FDA and Pfizer-BioNTech disclose that there are “no data” to support this claim.
- How the MDHHS blatantly lies that the scientific evidence “suggests natural immunity from COVID-19 may not last very long” when just the opposite is true, with studies having shown that natural immunity includes persistence of circulating antibodies, immunological memory and long-lived bone marrow plasma cells capable of rapidly ramping up antibody production in the event of re-exposure, and cellular immune responses that appear to be even more important than antibodies for protection against COVID-19.
- How the MDHHS tells parent that it’s safe for people who have already recovered from SARS-CoV-2 infection to get fully vaccinated, when in fact evidence of prior infection was an exclusion criterion in phase three trials and observational studies have consistently found that people with prior infection are at greater risk of experiencing adverse events from getting a COVID-19 vaccine, which some researchers have attributed to over-activation of the immune system.
Sources:
Michigan Department of Health and Human Services, Advertisement, “One Family’s Reason,” Nextdoor, Posted and accessed on December 7, 2021, https://nextdoor.com/p/j7XdK82KZBsQ.
Michigan State Government, “Kids COVID-19 Vaccine,” Michigan.gov, accessed December 7, 2021, https://www.michigan.gov/coronavirus/0,9753,7-406-98178_103214_109111—,00.html.
Michigan State Government, “COVID-19 Vaccine Questions and Answers for Parents,” Michigan.gov, November 3, 2021, accessed December 7, 2021, https://www.michigan.gov/documents/coronavirus/Parent_FAQs_5.14_Final_725378_7.pdf.
Jeremy R. Hammond, “Health Care Providers Mislead about Risks vs. Benefits of COVID-19 Vaccines for Children,” Jeremy R. Hammond.com, November 17, 2021, https://www.jeremyrhammond.com/2021/11/17/health-care-providers-mislead-about-risks-vs-benefits-of-covid-19-vaccines-for-children/.
Michigan State Government, “Statewide Available PPE and Bed Tracking,” Michigan.gov, updated December 6, 2021, accessed and archived December 7, 2021, https://web.archive.org/web/20211207201750/https://www.michigan.gov/coronavirus/0,9753,7-406-98159-523641–,00.html.
Michigan State Government, “Michigan Data,” Table: Demographics > Deaths, Michigan.gov, updated December 6, 2021, accessed December 7, 2021, https://www.michigan.gov/coronavirus/0,9753,7-406-98163_98173—,00.html.
Centers for Disease Control and Prevention, “Weekly Updates by Select Demographic and Geographic Characteristics: Provisional Death Counts for Coronavirus Disease 2019 (COVID-19),” Table 3: Comorbidities and other conditions, CDC.gov, data as of November 28, 2021, accessed December 7, 2021, https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/index.htm.
Centers for Disease Control and Prevention, “COVID-19 Vaccine Related Codes,” CDC.gov, reviewed November 19, 2021, accessed December 7, 2021, https://www.cdc.gov/vaccines/programs/iis/COVID-19-related-codes.html.
Food and Drug Administration, “Comirnaty and Pfizer-BioNTech COVID-19 Vaccine,” FDA.gov, updated November 23, 2021, accessed and archived December 7, 2021, https://web.archive.org/web/20211207203600/https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine.
Food and Drug Administration, “Vaccine Information Fact Sheet for Recipients and Caregivers about the Pfizer-BioNTech COVID-19 Vaccine to Prevent Coronavirus Disease 2019 (COVID-19) for Use in Individuals 5 Through 11 Years of Age,” FDA.gov, Revised October 29, 2021, accessed December 7, 2021, https://www.fda.gov/media/153717/download.
Food and Drug Administration, “Vaccine Information Fact Sheet for Recipients and Caregivers about the Pfizer-BioNTech COVID-19 Vaccine to Prevent Coronavirus Disease 2019 (COVID-19) for Use in Individuals 5 Through 11 Years of Age,” FDA.gov, Revised June 25, 2021, archived August 1, 2021, https://web.archive.org/web/20210801001409/https://www.fda.gov/media/144414/download.
Department of Health and Human Services, “Declaration Under the Public Readiness and Emergency Preparedness Act for Medical Countermeasures Against COVID-19,” Federal Register, March 17, 2020, https://www.federalregister.gov/documents/2020/03/17/2020-05484/declaration-under-the-public-readiness-and-emergency-preparedness-act-for-medical-countermeasures.
Emmanuel B. Walter et al., “Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age,” New England Journal of Medicine, November 9, 2021, https://doi.org/10.1056/NEJMoa2116298.
Food and Drug Administration, “Fact Sheet for Healthcare Providers Administering Vaccine (Vaccination Providers): Emergency Use Authorization (EUA) of the Pfizer-BioNTech COVID-19 Vaccine to Prevent Coronavirus Disease 2019 (COVID-19) for 5 through 11 Years of Age,” FDA.gov, revised October 29, 2021, accessed December 7, 2021, https://www.fda.gov/media/153714/download.
Jeremy R. Hammond, “Fact Checking the ‘Fact Checkers’ on Natural Immunity to SARS-CoV-2,” JeremyRHammond.com, November 29, 2021, https://www.jeremyrhammond.com/2021/11/29/fact-checking-the-fact-checkers-on-natural-immunity-to-sars-cov-2/.
Jeremy R. Hammond, “The Superiority of Natural Immunity to SARS-CoV-2,” JeremyRHammond.com, updated November 5, 2021, https://www.jeremyrhammond.com/natural-immunity-to-sars-cov-2/.
Jackson S. Turner et al., “SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans,” Nature, May 24, 2021, https://doi.org/10.1038/s41586-021-03647-4.
Ewen Callaway, “Had COVID? You’ll probably make antibodies for a lifetime,” Nature, May 26, 2021, https://doi.org/10.1038/d41586-021-01442-9.
La Jolla Institute for Immunology, “T cells take the lead in controlling SARS-CoV-2 and reducing COVID-19 disease severity,” LJI.org, September 16, 2020, https://www.lji.org/news-events/news/post/t-cells-take-the-lead-in-controlling-sars-cov-2-and-reducing-covid-19-disease-severity/.
Catherine J. Reynolds et al., “Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose,” Science, June 25, 2021, https://doi.org/10.1126/science.abh1282.
Fernando P. Polack et al., “Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine,” New England Journal of Medicine, December 31, 2020, https://doi.org/10.1056/NEJMoa2034577.
Alexander G. Mathioudakis et al., “Self-Reported Real-World Safety and Reactogenicity of COVID-19 Vaccines: A Vaccine Recipient Survey,” Life, March 17, 2021, https://doi.org/10.3390/life11030249.
Florian Krammer et al., “Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine,” New England Journal of Medicine, March 10, 2021, https://doi.org/10.1056/NEJMc2101667.
Cristina Menni et al., “Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study,” Lancet Infectious Diseases, April 27, 2021, https://doi.org/10.1016/S1473-3099(21)00224-3.
Rachael Kathleen Raw et al., “Previous COVID-19 infection, but not Long-COVID, is associated with increased adverse events following BNT162b2/Pfizer vaccination,” Journal of Infection, May 29, 2021, https://doi.org/10.1016/j.jinf.2021.05.035.
Antonella d’Arminio Monforte et al., “Association between previous infection with SARS CoV-2 and the risk of self-reported symptoms after mRNA BNT162b2 vaccination: Data from 3,078 health care workers,” EClinicalMedicine, May 30, 2021, https://doi.org/10.1016/j.eclinm.2021.100914.
Amanda K. Debes et al., “Association of Vaccine Type and Prior SARS-CoV-2 Infection With Symptoms and Antibody Measurements Following Vaccination Among Health Care Workers,” JAMA Internal Medicine, August 16, 2021, https://doi.org/10.1001/jamainternmed.2021.4580.
Shai Efrati et al., “Safety and humoral responses to BNT162b2 mRNA vaccination of SARS-CoV-2 previously infected and naive populations,” Scientific Reports, August 16, 2021, https://doi.org/10.1038/s41598-021-96129-6.
Centers for Disease Control and Prevention, “Epidemiology of COVID-19 in Children Aged 5-11 Years,” presented to the FDA Vaccine and Related Biological Products Advisory Committee (VRPAC) meeting on October 26, 2021, accessed December 7, 2021, https://www.fda.gov/media/153508/download.
Sara Y Tartof et al., “Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study,” The Lancet, October 4, 2021, https://doi.org/10.1016/S0140-6736(21)02183-8.
Peter Nordström et al., “Effectiveness of Covid-19 Vaccination Against Risk of Symptomatic Infection, Hospitalization, and Death Up to 9 Months: A Swedish Total-Population Cohort Study,” Preprints with THE LANCET, SSRN, October 25, 2021, https://dx.doi.org/10.2139/ssrn.3949410.
Centers for Disease Control and Prevention, “Interim Public Health Recommendations for Fully Vaccinated People,” CDC.gov, updated November 19, 2021, accessed December 7, 2021, https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated-guidance.html.
Aaron Siri, “CDC Admits Crushing Rights of Naturally Immune Without Proof They Transmit the Virus,” Injecting Freedom, Substack, November 11, 2021, https://aaronsiri.substack.com/p/cdc-admits-crushing-rights-of-naturally.



Thanks Jeremy – nice work!
Have you sent your article back to Michigan state health department and asked them for a response?
Australia has authorised use of Pfizer paediatric vaccine in 5 – 11 year olds and will start rolling out on January 10th.
I am compiling a paper on ‘Risk-Benefit Analysis of COVID-19 mRNA Provisionally Approved Vaccines for Use in Under 18s’ and have got some great reference from your work – thank you. Happy to send onto you when I am done.
Keep making a difference!
Love Kathy
Thanks, Kathy. I have not contacted MDHHS for comment. It’s taken cease and desist letters from lawyers to get MDHHS to stop spreading disinformation previously, i.e., their lie that the vaccines were FDA “approved” when all were FDA unapproved. I’m hoping others will build on my work by taking such legal actions.
Jeremy…you are so intellectual. This message will not resonate with mediocre minds. Only a marginal few will go the hour and then defer from getting their kids jabbed. Which may be ok. Yes, you will “save” a small appreciative future generation. Alas, consensus think groupies will lead the lemmings over the cliff. Sorry. It is an evolutionary event…most will be excised from the gene pool. Hard words, I know. Maybe you too suspect this consequence? I marvel at your depth of intelligence. Please keep up your work.
Yes, I do suspect that consequence. But if I can save one, it will have been worth the effort.